38 h2y acid or base
PSW | PDF - Scribd psw.txt - Free ebook download as Text File (.txt), PDF File (.pdf) or read book online for free. Trending Articles about h2y acid or base | Echemi Find everything about h2y acid or base you need.You can dig into the news and opinion of h2y acid or base on echemi.com. ... Fluid, Electrolyte, and Acid-base Balance. Vitamins and Minerals Medicines. Digestive System Drugs. Blood System Drugs. Circulatory System Drugs. Diagnostic Agents. Specialty Drugs. Drug Metabolism.
› doc › 207631660Dictionary | PDF - Scribd Dictionary - Free ebook download as Text File (.txt), PDF File (.pdf) or read book online for free. This is a dictionary file with all the words ever
H2y acid or base
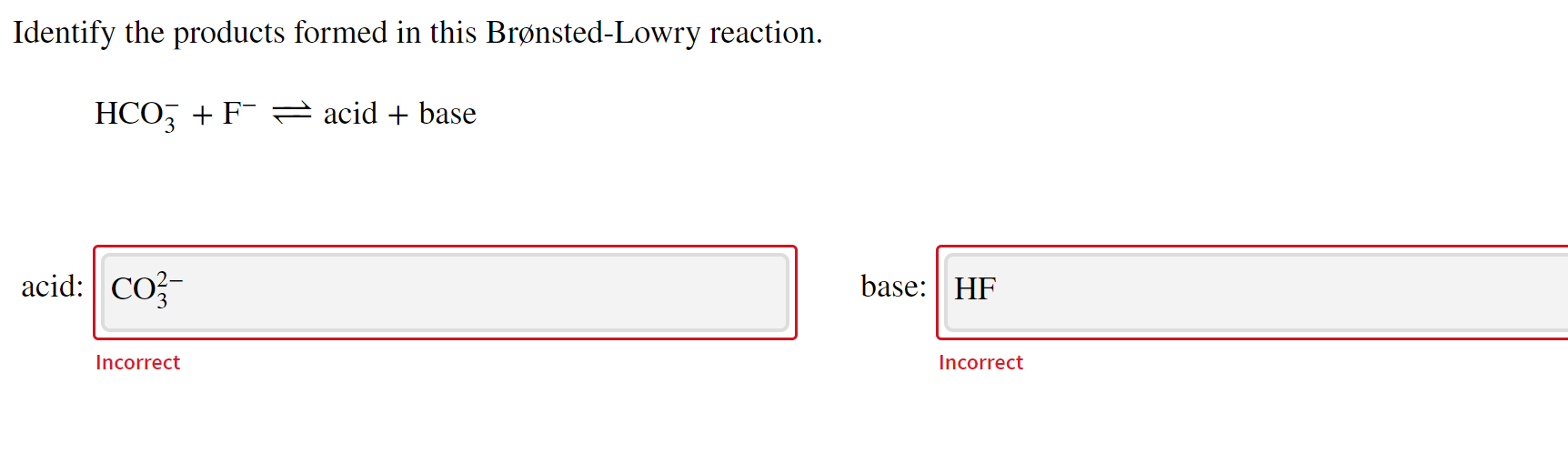
Chem 121 Exam 3 Flashcards | Quizlet Classify each reactant and product in this reaction as an acid or base according to the Brøsted theory HF, H2O, F-, H3O+ ... H2Y-: base H2Z-: acid H3Y: acid HZ2-: base. Identify the conjugate acid for each base. - conjugate acid of HSO−4: - conjugate acid of SO−4^2-- conjugate acid of NH3. H2SO4 HSO4-NH4+ Identify the pair of species that ... Is Hydrofluoric Acid (HF) a Strong or Weak Acid? - ThoughtCo Hydrofluoric acid is the only hydrohalic acid (such as HCl, HI) that is not a strong acid. HF ionizes in an aqueous solution like other acids: . HF + H 2 O ⇆ H 3 O + + F -. Hydrogen fluoride does actually dissolve fairly freely in water, but the H 3 O + and F - ions are strongly attracted to each other and form the strongly bound pair, H 3 O ... Identify each reactant and product in this reaction as a Brønsted acid ... conjugate acid of CO2−3: conjugate acid of NH3: Give the formula of the conjugate base of HSO−4. conjugate base: Give the formula for the conjugate acid of HSO−4. conjugate acid: What is the hydrogen ion concentration of a solution with pH=11.25? dentify the products formed in this Brønsted-Lowry reaction. HSO−4+HBrO↽−−⇀acid+base
H2y acid or base. H2Y- Acid Or Base : A Novel Amperometric Nitric Oxide Sensor Based On ... H2Y- Acid Or Base : A Novel Amperometric Nitric Oxide Sensor Based On Imprinted Microenvironments For Controlling Metal Coordination Sciencedirect. 1 answer to label each reactant and product in this reaction as a bronsted acid or base. Conjugate pairs h2y base acid h3y h2z acid base h3 2. H2Y- Acid Or Base / Identify The Conjugate - Dika Hermawan H2Y- Acid Or Base / Identify The Conjugate This is a dictionary file with all the words ever The breakdown of this figure by category is shown in figure 28. Our shareholder base is widely diversified, with approximately 39 per cent of shares held in australia, 30 per cent in europe, 18 per cent in north america, 8 per cent in south africa and 5 ... Answered: Identify each reactant and product in… | bartleby Identify each reactant and product in this reaction as a Brønsted acid or base. H2Y−+H2Z−↽−−⇀H3Y+HZ2−. › Archives › edgarOur New Approach to Reporting - SEC Our shareholder base is widely diversified, with approximately 39 per cent of shares held in Australia, 30 per cent in Europe, 18 per cent in North America, 8 per cent in South Africa and 5 per cent in Asia. Globally, the Company spent in the order of US$12.5 billion sustaining its businesses.
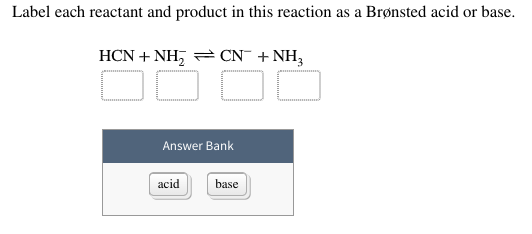
Is H2O an acid or a base? - Answers Best Answer. Copy. H2O can be either or a base or an acid. This is known as being amphiprotic, which means that this substance can either donate or accept an electron. When water donates an ... EOF Solved Identify each reactant and product in this reaction - Chegg This problem has been solved! See the answer Identify each reactant and product in this reaction as a Brønsted acid or base. H2Y−+H2Z−↽−−⇀H3Y+HZ2−H2Y−+H2Z−↽−−⇀H3Y+HZ2− Expert Answer 95% (57 ratings) Given reaction is H2Y- + H2Z- -------> H3Y + HZ2- As we know pr … View the full answer Previous question Next question Solved Label each reactant and product in this reaction as a - Chegg This problem has been solved! See the answer. See the answer See the answer done loading. Label each reactant and product in this reaction as a Bronsted acid or base. H2Y- + H2Z- <===> H3Y + HZ2-. Expert Answer. Who are the experts?
In the reaction between H2Y - and H2Z - , if H2Y - acts like a base ... Label each reactant and product in this reaction as a Bronsted acid or base. H2Y- + H2Z- <===> H3Y + HZ2- Posted one year ago. 1. Determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases). 2. Use the curved-arrow formalism to show the movement of electron pairs in these reactions, as well as the ... Answered: In the reaction between H2Y - and H2Z -… | bartleby In the reaction between H2Y - and H2Z - , if H2Y - acts like a base, and H2Z - acts like an acid, the products of the reaction are: A) H3Y - and HZ- B) HY- and H3Z - C) H3Y and HZ2- D) H2Y and HZ- E) H3Y and HZ › 37327304 › Qui_mica_Anali_tica_6(PDF) Química Analítica, 6ta Edicion - Academia.edu Química Analítica, 6ta Edicion - Gary D. Christian Is H2O an acid or base or both? Its conjugate acid-base pairs - Water In presence of a strong base like NaOH, KOH, NH 3, etc. the H 2 O will act as an acid. In presence of a strong acid like HCl, HNO 3, H 2 SO 4, etc. the H 2 O will act as a base. But H 2 O can never be both acid or base at the same time. We can't say H 2 O (water) is absolute acid or base or neutral in nature as it totally depends on whom it reacts.
H2Y- Acid Or Base - 2 | Haryati Oman H2Y- Acid Or Base - 2. Label each reactant and product in this reaction as a bronsted acid or base. Find out how to calculate the amount of an acid of known concentration needed to neutralize a base of known concentration for a neutral ph balance. B contains 3.90 g of naoh per dm3 of solution.
Solved Identify each reactant and product in this reaction - Chegg Chemistry questions and answers. Identify each reactant and product in this reaction as a Brønsted acid or base. HY + H, Z =HAY + HZ- base base acid base Answer Bank base acid Calculate [H], [C107), and [OH") in an aqueous solution that is 0.145 M in HCIO, (aq) at 25 °C. 130 M Incorrect .130 (CIO) = M Incorrect JOH") - 7.69 x10-14 M Incorrect ...
Acid vs Base - Difference and Comparison | Diffen Bases are the chemical opposite of acids. Acids are defined as compounds that donate a hydrogen ion (H +) to another compound (called a base).Traditionally, an acid (from the Latin acidus or acere meaning sour) was any chemical compound that, when dissolved in water, gives a solution with a hydrogen ion activity greater than in pure water, i.e. a pH less than 7.0.
H2Y Acid Or Base - Plos One Molecular Characterisation Of Genital Human ... H2Y Acid Or Base - Plos One Molecular Characterisation Of Genital Human Papillomavirus Among Women In Southwestern Nigeria Label each reactant and product in this reaction as a brønsted acid or base. Label each reactant and product in this reaction as a bronsted acid or base. Acid, h2y, with the standardized naoh solution.
H2Y Acid Or Base - Bronsted Lowry Acid And Base Chemistry Video Clutch ... There's going to be an acid and base on the reactant side and an. (e) the graph below shows the results obtained by titrating a different weak acid, h2y, with the standardized naoh solution. There's going to be an acid and base on the reactant side and an. Free answer to label each reactant and product in this reaction as a bronsted acid or ...
Dictionary | PDF - Scribd Dictionary - Free ebook download as Text File (.txt), PDF File (.pdf) or read book online for free. This is a dictionary file with all the words ever
› doc › 257567726PSW | PDF - Scribd psw.txt - Free ebook download as Text File (.txt), PDF File (.pdf) or read book online for free.
Our New Approach to Reporting - SEC Our shareholder base is widely diversified, with approximately 39 per cent of shares held in Australia, 30 per cent in Europe, 18 per cent in North America, 8 per cent in South Africa and 5 per cent in Asia. ... the emissions of sulphuric acid mist have been significantly reduced. A preventive approach to managing our health issues will focus ...
Identify each reactant and product in this reaction as a Brønsted acid ... conjugate acid of CO2−3: conjugate acid of NH3: Give the formula of the conjugate base of HSO−4. conjugate base: Give the formula for the conjugate acid of HSO−4. conjugate acid: What is the hydrogen ion concentration of a solution with pH=11.25? dentify the products formed in this Brønsted-Lowry reaction. HSO−4+HBrO↽−−⇀acid+base


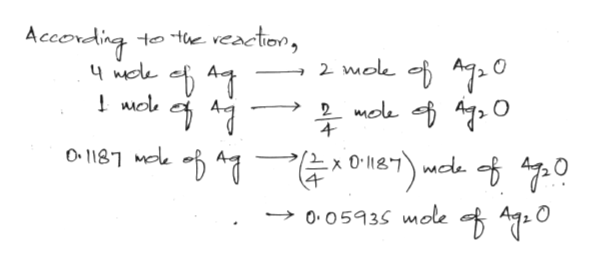


Post a Comment for "38 h2y acid or base"