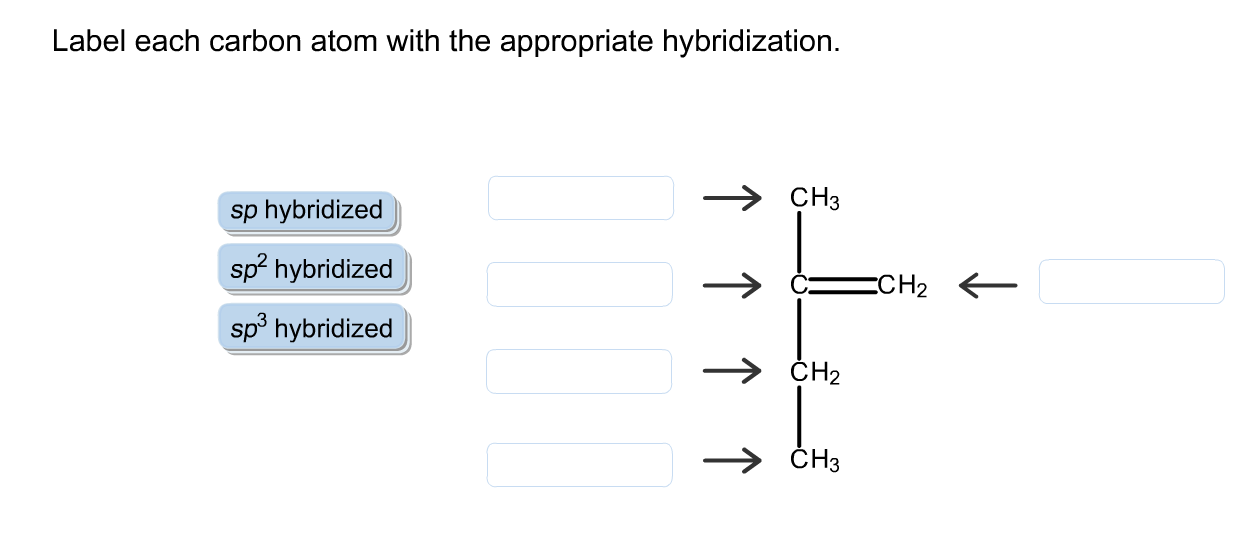
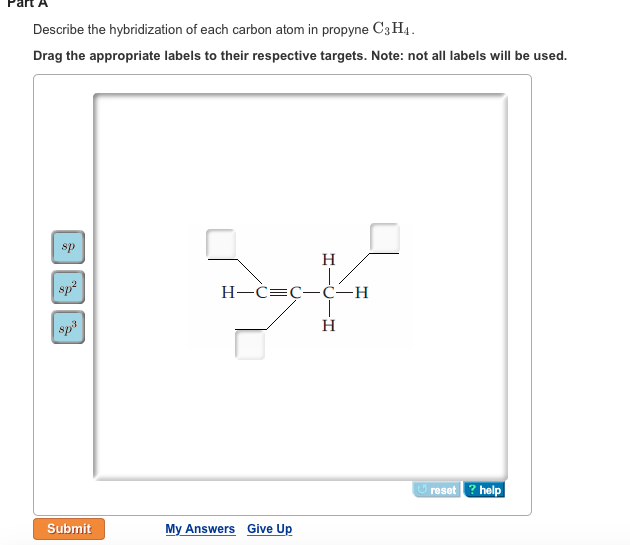
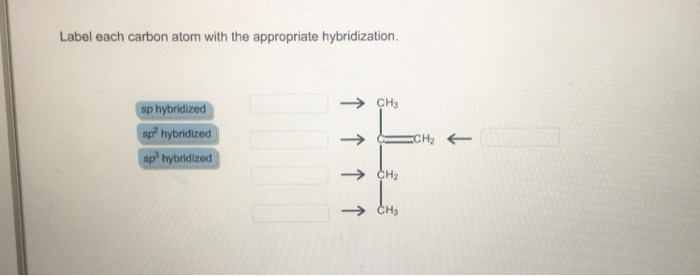
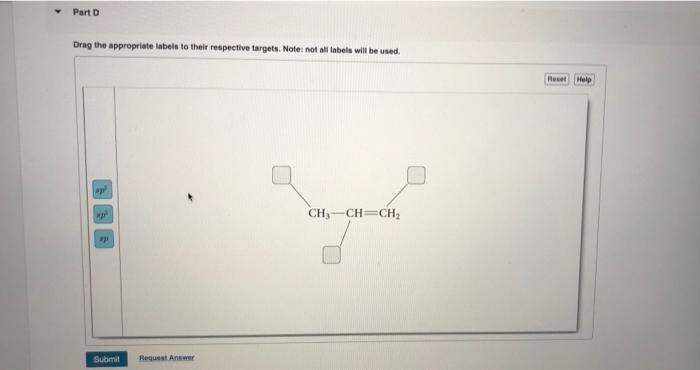
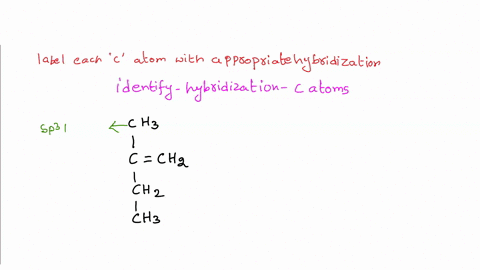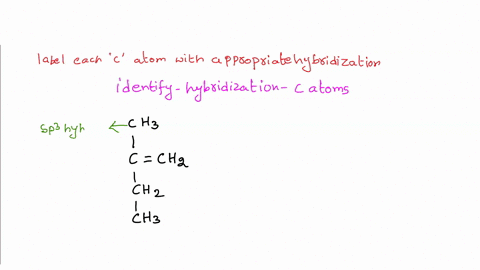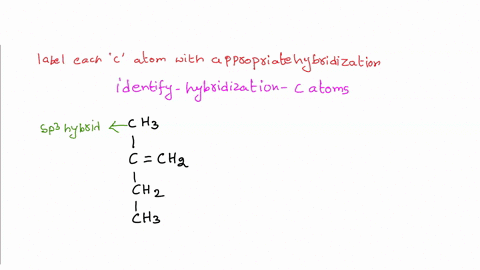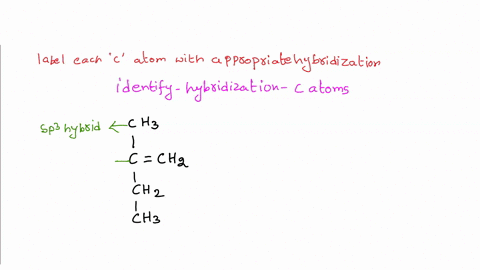
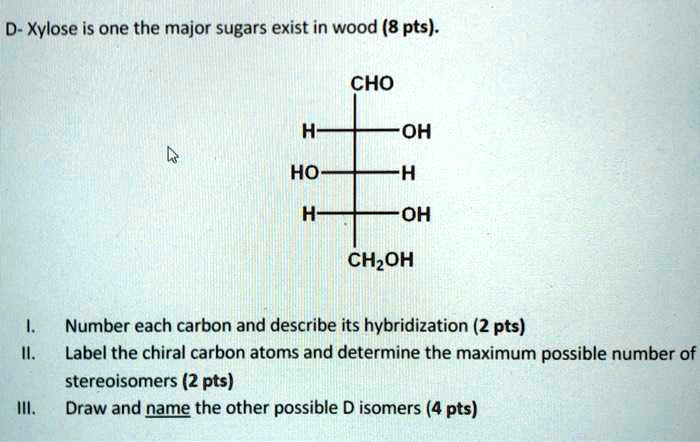
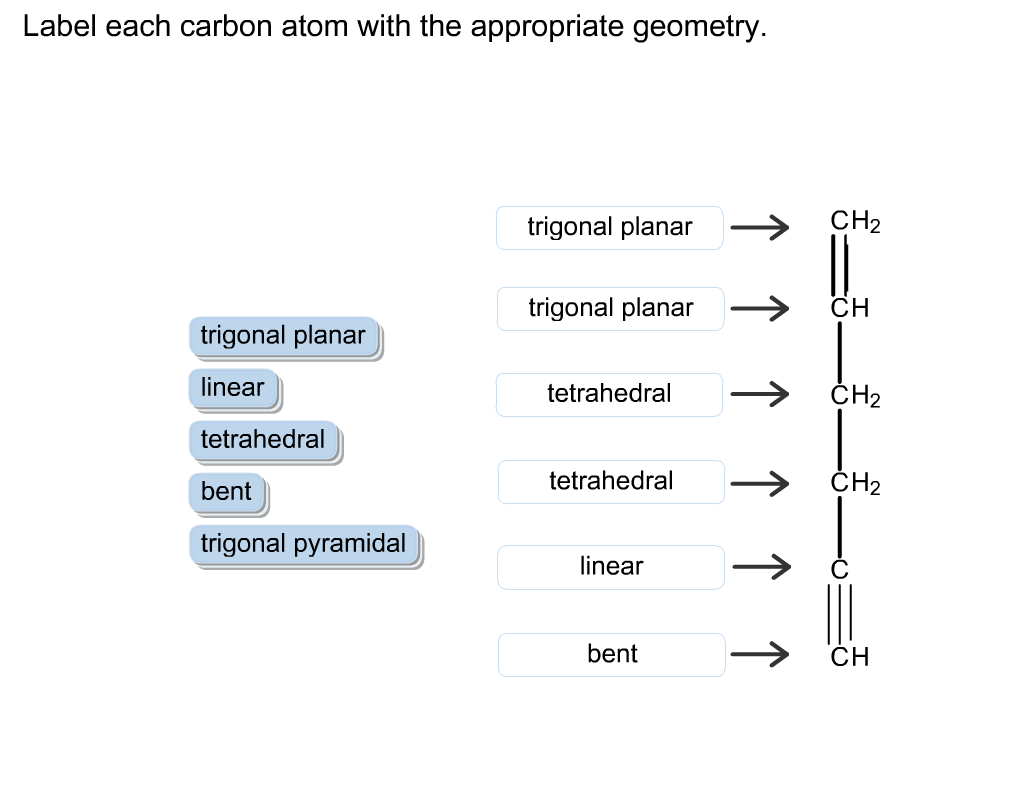
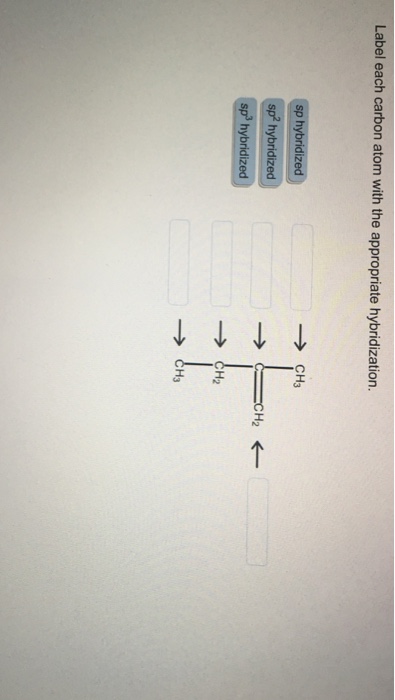
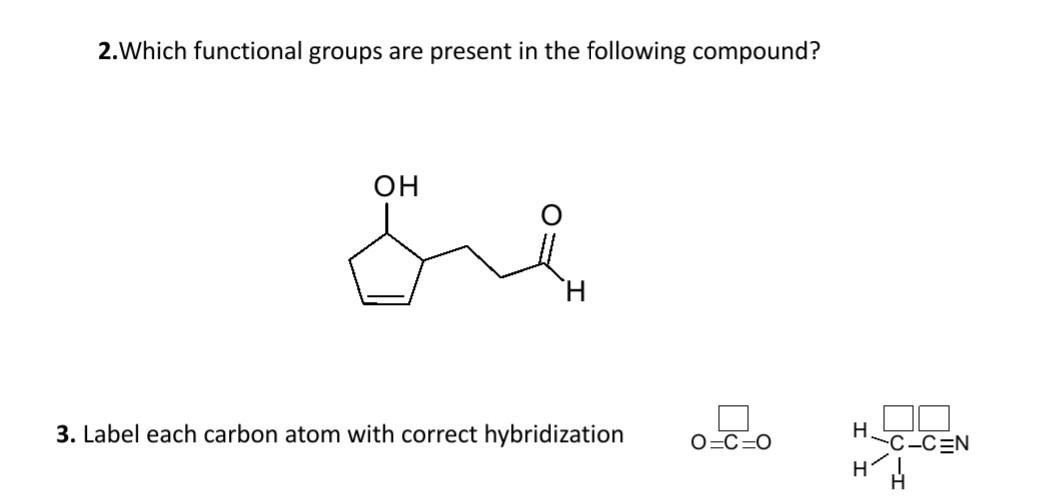
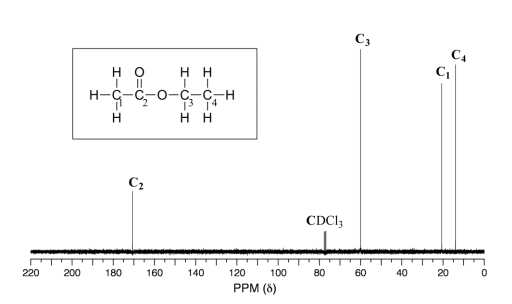
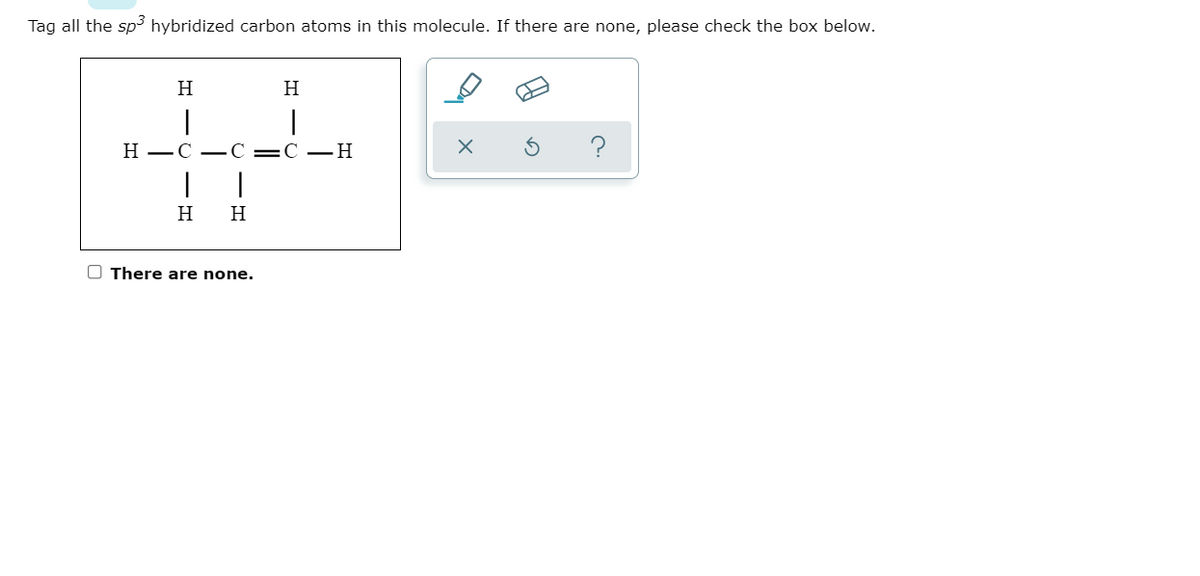
39 label each carbon atom with the appropriate hybridization
en.wikipedia.org › wiki › Lone_pairLone pair - Wikipedia The familiar alkynes have a carbon-carbon triple bond (bond order 3) and a linear geometry of 180° bond angles (figure A in reference ). However, further down in the group ( silicon , germanium , and tin ), formal triple bonds have an effective bond order 2 with one lone pair (figure B [19] ) and trans -bent geometries. › questions-and-answers › determineAnswered: Determine if the bond between each… | bartleby Transcribed Image Text: Part A Determine if the bond between each pairs of atoms would be pure covalent, polar covalent, or ionic Drag the appropriate items to their respective bins. Reset Help Nand N K and F I and Cl C and O Pure covalent Polar covalent lonic
› topics › engineeringMetasurface - an overview | ScienceDirect Topics Each conformal metasurface was composed of a layer of 4 × 4 radiating patches loaded with symmetrically extended open-ended U-slots and a reflector layer. The reflector layer of 4 × 4 square patches was designed to exhibit a negative-index feature. A mushroom-like electromagnetic band-gap (EBG) unit cell array was arranged around the feeding ...
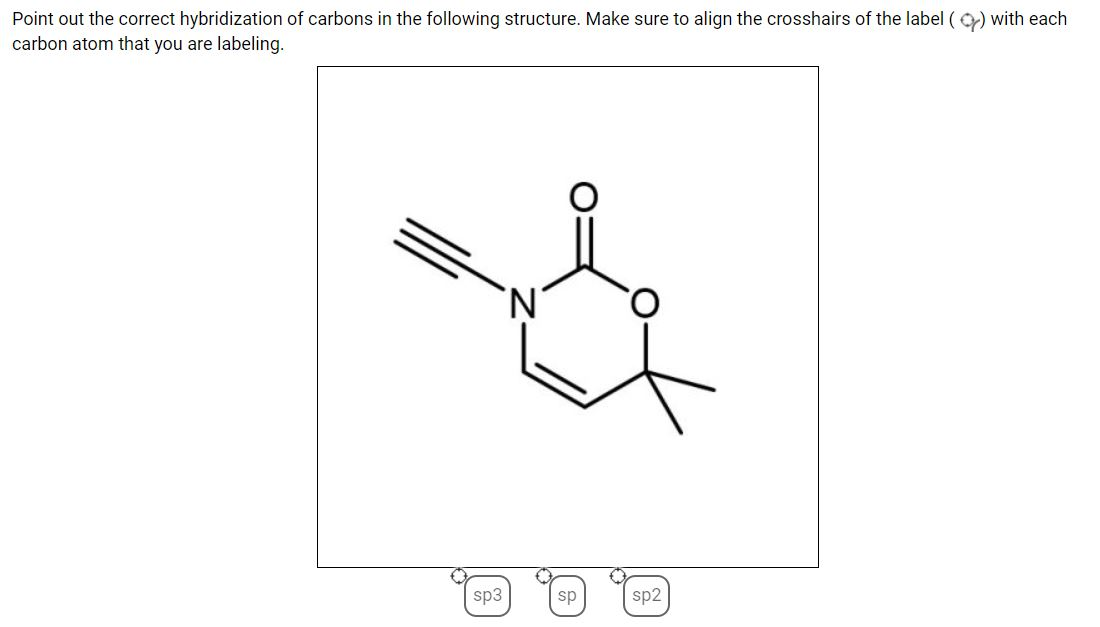
Label each carbon atom with the appropriate hybridization
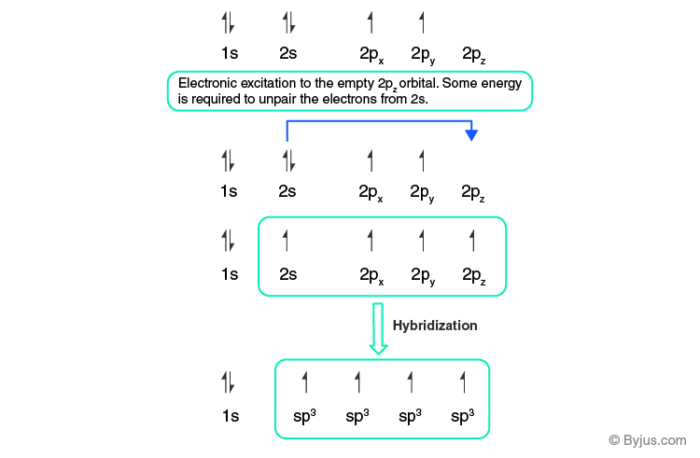
› 48903430 › Inorganic_Chemistry_4Inorganic Chemistry 4th edition, Catherine Housecroft Enter the email address you signed up with and we'll email you a reset link. › questions-and-answers › prove-whyAnswered: Prove why the molecular identity of BF3… | bartleby A: Solution- sp³ hybridization - • In this type of hybridization one's' orbital of first atom combines… Q: Write the best Lewis dot structure for NH₄⁺, being sure to give the electronic geometry, molecular… en.wikipedia.org › wiki › GrapheneGraphene - Wikipedia Each atom in a graphene sheet is connected to its three nearest neighbors by a strong σ-bond, and contributes to a valence band one electron that extends over the whole sheet. This is the same type of bonding seen in carbon nanotubes and polycyclic aromatic hydrocarbons , and (partially) in fullerenes and glassy carbon .
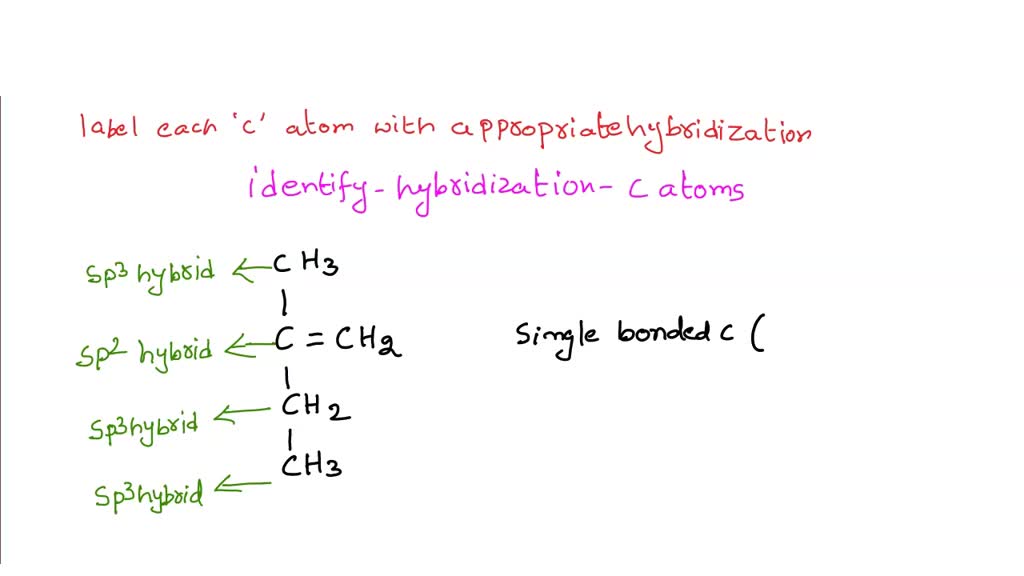
Label each carbon atom with the appropriate hybridization. › articles › s41467/021/27071-4Photoluminescence mechanism of carbon dots: triggering high ... Nov 25, 2021 · Carbon dots (CDs), an emerging, highly-promising type of fluorescent carbon-based nanomaterials, have attracted tremendous research attention in diverse applications due to the fascinating merits ... en.wikipedia.org › wiki › GrapheneGraphene - Wikipedia Each atom in a graphene sheet is connected to its three nearest neighbors by a strong σ-bond, and contributes to a valence band one electron that extends over the whole sheet. This is the same type of bonding seen in carbon nanotubes and polycyclic aromatic hydrocarbons , and (partially) in fullerenes and glassy carbon . › questions-and-answers › prove-whyAnswered: Prove why the molecular identity of BF3… | bartleby A: Solution- sp³ hybridization - • In this type of hybridization one's' orbital of first atom combines… Q: Write the best Lewis dot structure for NH₄⁺, being sure to give the electronic geometry, molecular… › 48903430 › Inorganic_Chemistry_4Inorganic Chemistry 4th edition, Catherine Housecroft Enter the email address you signed up with and we'll email you a reset link.
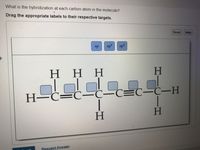
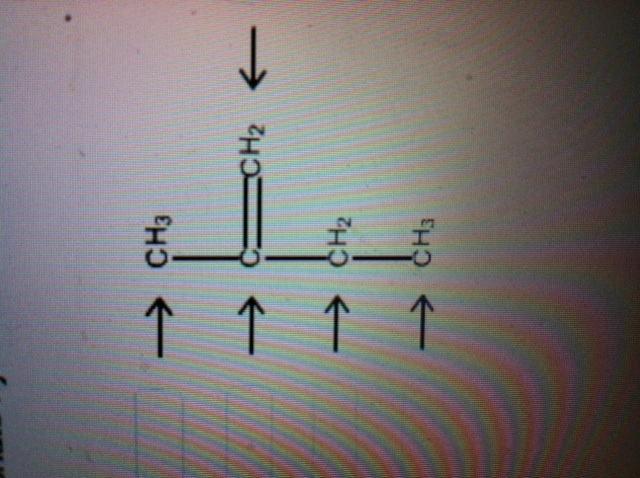
label each carbon atom with the appropriate hybridization ch answer bank sp hybridized chz hybridized ch hyhridved ch 12818

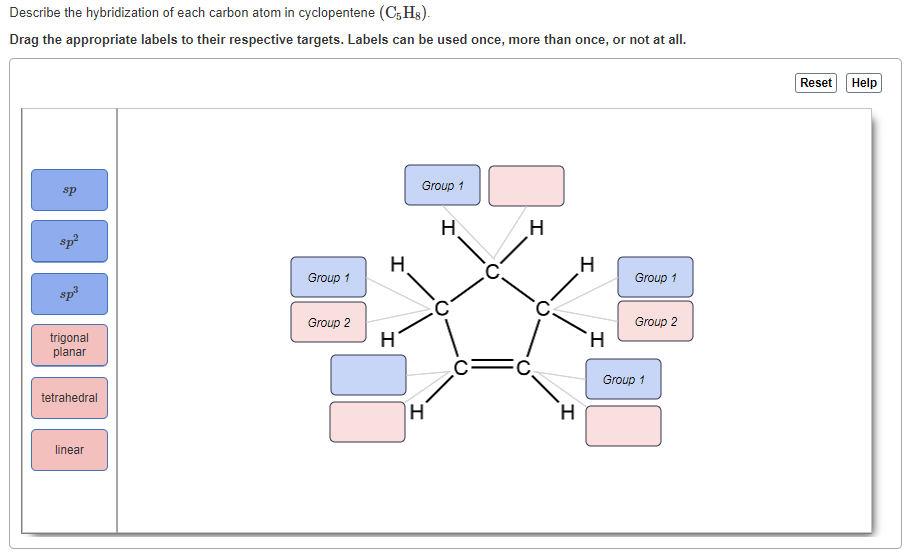












Post a Comment for "39 label each carbon atom with the appropriate hybridization"